Transport of Respiratory Gases
Introduction : Transport of Respiratory Gases
Transport of Respiratory Gases- Hemoglobin and oxygen (O 2) are nearly completely carried to the tissue capillaries when oxygen (O 2) diffuses from the alveoli into the pulmonary circulation. Red blood cells contain hemoglobin, which enables the blood to carry 30–100 times the amount of oxygen as compared to oxygen dissolved in blood water.O2 combines with a variety of nutrients in the body’s tissue cells to produce a significant amount of carbon dioxide (CO2). This CO2 is taken up by tissue capillaries and then exhaled back into the lungs. Similar to oxygen, carbon dioxide also binds to blood components, causing a 15–20 fold increase in CO2 transport. It is found that just 0.3 ml of arterial blood—or around 2 percent—is present in the physical solution in plasma water, and that around 98% of the oxygen in blood is chemically bonded to hemoglobin. However, the oxygen pressure in plasma is caused by this tiny amount in the physical solution. Venous blood contains 52% CO2 per 100 ml, but arterial blood has approximately 48% CO2. The CO2 pressure of venous and arterial blood is 40 mmHg and 46 mmHg, respectively.
1. Oxygen Transport :
Oxygen is transported by the blood from the alveoli to the tissues in two forms:
A. via substances dissolved in the bloodstream
B. bound to hemoglobin.
A. Via substances dissolved in the bloodstream
Transport of Respiratory Gases– In plasma water, oxygen dissolves and is carried in this physical form. For every 100 milliliters of plasma, this technique hardly transmits 0.3 milliliters of oxygen. It only makes up about 3% of the total oxygen in your blood. The low solubility of oxygen in plasma water is the cause of this. Nevertheless, this kind of oxygen transfer is necessary to meet the high oxygen needs of tissues in situations like muscular training. The oxygen pressure in the plasma, however, is caused by this tiny amount in the physical solution. The oxygen pressure in the blood is also responsible for the transfer of this gas from the alveolar air to the venous blood across the alveolar-capillary membrane and also for the transfer of this gas from the arterial blood to the tissue fluid across the capillary membrane. Furthermore, it is the oxygen pressure in the plasma that controls the amount of oxyhemoglobin present in the blood.
B. bound to hemoglobin
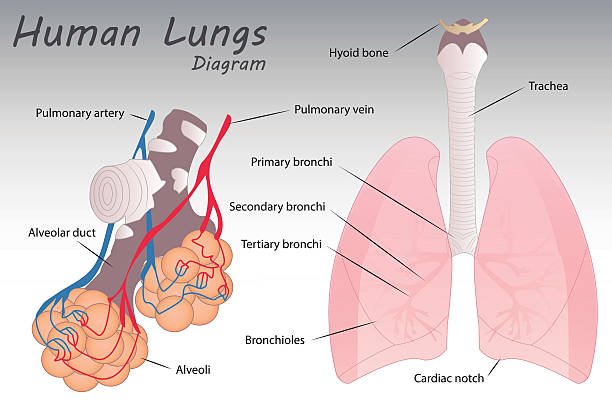
Transport of Respiratory Gases- Hemoglobin and oxygen combine in the blood to form oxyhemoglobin, which is carried by the oxygen. This kind of oxygen transportation is crucial since it carries the majority of oxygen—97%—in its whole. Hemoglobin and oxygen bond to each other physically exclusively; this is called oxygen enrichment, not oxidation. There are various benefits to this technique of oxygen binding to hemoglobin: Hemoglobin may readily release oxygen as needed. Hemoglobin readily absorbs oxygen when the blood’s partial pressure of oxygen is high. When the blood’s partial pressure of oxygen decreases, hemoglobin releases oxygen. The iron in the heme portion of hemoglobin is bound by oxygen. A molecule of hemoglobin has four iron atoms in it. Hemoglobin contains iron in its ferrous state. One oxygen molecule is bonded to every iron atom. Iron only exists in the form of iron(II) after binding. That’s why oxygenation, rather than oxidation, refers to the process of oxygen attaching to hemoglobin.
Hemoglobin Oxygen Carrying Capacity :-
In Transport of Respiratory Gases , The amount of oxygen carried by one gram of hemoglobin is known as its oxygen carrying capacity. 1.34 milliliters per gram is the value. The quantity of oxygen that blood can carry is referred to as its oxygen carrying capacity. Blood has a normal hemoglobin content of 15%. Blood with 15% hemoglobin must carry 20.1 ml% oxygen because hemoglobin has an oxygen carrying capacity of 1.34 ml/g. There is 20.1ml of oxygen in 100ml of blood. However, blood containing 15g% hemoglobin transports only 19 mL% oxygen. 19 mL of oxygen are transported by 100 mL of blood. Blood’s ability to carry oxygen is limited to 19 milliliters due to the fact that hemoglobin is only approximately 95% saturated with oxygen.
Oxygen-Hemoglobin Dissociation Curve :-
In Transport of Respiratory Gases, Under normal conditions, the oxygen-hemoglobin dissociation curve is S-shaped or sigmoidal. The lower part of the curve shows the dissociation of oxygen from hemoglobin. The upper part of the curve shows the oxygen uptake of hemoglobin as a function of oxygen partial pressure.
Oxyhemoglobin Dissociation Curve P50 Value: The partial pressure of oxygen (P50) at which hemoglobin reaches 50% oxygen saturation is known. Hemoglobin is around 50% saturated at 25–27 mmHg, the partial pressure of oxygen. This indicates that the blood’s oxygen content is 50%. Saturation is 75% at 40 mmHg of oxygen pressure. Saturation is 95% at 100 mmHg of oxygen pressure.
Oxygen-Hemoglobin Dissociation Curve's Influential Factors :-
The Oxyhemoglobin Dissociation Curve’s Influential Factors Several causes cause the oxyhemoglobin dissociation curve to move left or right.
1. A movement to the left denotes hemoglobin’s absorption (binding) of oxygen.
2. A movement to the right denotes hemoglobin and oxygen breaking apart.
1. Movement to the right: The oxyhemoglobin dissociation curve shifts to the right under the following conditions:
i. A decrease in the oxygen partial pressure
ii. A rise in the partial pressure of carbon dioxide (Bohr effect)
iii. A rise in the concentration of hydrogen ions and a fall in pH (acid content)
iv. Raising body temperature
v. Red blood cell excess 2,3-diphosphoglycerate (DPG).
2. Movement to the left- The following circumstances cause the oxyhemoglobin dissociation curve to move to the left:
i. Compared to adult hemoglobin, fetal hemoglobin has a higher affinity for oxygen in blood.
ii. The pH (alkalinity) rises as the concentration of hydrogen ions falls.
Bohr Effect :- in Transport of Respiratory Gases, The Bohr effect is the phenomenon wherein hemoglobin’s affinity for oxygen is decreased when carbon dioxide is present. In tissues, due to ongoing metabolic activity, the partial pressure of carbon dioxide is very high and the partial pressure of oxygen is low. This pressure gradient causes carbon dioxide to enter the blood and oxygen to be released from the blood to the tissues. The oxygen-binding affinity of hemoglobin is decreased in the presence of carbon dioxide. Carbon dioxide further increases the supply of oxygen to the tissues, shifting the oxygen dissociation curve to the right. Factors Affecting the Bohr Effect Any factor that shifts the oxygen dissociation curve to the right (see above) will increase the Bohr effect.
Advantages of the Sigmoid Curve :-
1. This waveform is such that with normal alveolar p02, blood leaving the lungs is almost completely saturated with hemoglobin. A. Further increase in alveolar p02 under normal circumstances. B. Inhaling O2 offers no advantage in terms of hemoglobin saturation.
2. The flat upper part of the curve indicates that there is only a relatively small decrease in hemoglobin saturation if the partial pressure of oxygen in the alveolar air does not fall below 60 mmHg.
3. The rapid increase in the oxygen dissociation curve in the lower oxygen partial pressure range indicates that at the oxygen partial pressures prevailing in the tissues (40-20 mmHg), there is rapid breakdown of oxy-Hb and consequent release of O2.
Increases in CO2, pH, temperature, and DPG levels all promote rapid release of oxygen to active tissues.
2. Carbon Dioxide Transport :
Transport of Respiratory Gases- Because CO2 may often be delivered in far larger quantities than O2, even under the most aberrant situations, CO2 transport through the blood is less difficult than O2 transport. Nonetheless, there is a strong correlation between the blood’s CO2 levels and the bodily fluids’ acid-base balance. During a typical resting state, the lungs get 4 milliliters of CO2 on average for every 100 milliliters of blood that leaves the tissues.
Carbon dioxide is transported from the cells to the alveoli by the blood. The blood carries carbon dioxide in three different ways:
1. Soluble form (7%).
2. As 63 percent bicarbonate
3. As (30%) carbamino compounds
1. Soluble form (7%) :-
Transport of Respiratory Gases- A tiny amount of the CO2 is carried in dissolved form to the lungs. It should be noted that arterial blood has a PCO2 of 40 mmHg and venous blood has a PCO2 of 45 mmHg. In the blood, at 45 mmHg, there is roughly 2.7 ml/dl (2.7 volume percent) of dissolved CO2. At 40 mmHg, there is a 0.3 ml, or approximately 2.4 ml, difference in the amount of dissolved CO2. Therefore, only about 0.3 ml of dissolved CO2 are delivered per 100 ml of bloodstream. This amounts to roughly 7% of the total CO2 that is typically transferred.
2. As 63 percent bicarbonate :-
Transport of Respiratory Gases- Bicarbonate is used to transport about 63% of carbon dioxide. The red blood cells take up carbon dioxide from the plasma. Carbon dioxide and water interact in red blood cells to generate carbonic acid. The carbonic anhydrase present in red blood cells causes the process to proceed significantly more quickly. The reaction is accelerated by this enzyme. Plasma does not contain carbonic anhydrase; only red blood cells do. Red blood cells therefore produce at least 200–300 times more carbon dioxide than plasma does.
The acid carbonic is highly unstable. Bicarbonate and hydrogen ions are the end products of nearly all (99.9%) of the carbonic acid generated in red blood cells. Within the cells, the concentration of bicarbonate ions keeps rising. Because of the high concentration, bicarbonate ions diffuse through the cell membrane into the plasma.
Dissociation of Carbonic Acid :-
In Transport of Respiratory Gases, Carbonic acid (H2CO3) formed in red blood cells dissociates into H+ and bicarbonate ions (H+ and HCO3-) in a fraction of a second. Due to the hemoglobin protein’s potent acid-base buffering properties, the majority of H+ ions in red blood cells bind to it. Subsequently, the HCO3- ions leave the red blood cells in higher proportion and disperse into the plasma, to be replaced by the chloride ions upon entering the red blood cells. This diffusion is made possible by the presence of special bicarbonate-chloride carrier proteins in the red blood cell membrane, which transport these two ions in the opposite direction at high speed. Thus, the chloride content of venous red blood cells is higher than that of arterial red blood cells, a phenomenon called chloride shift. The reversible binding of CO2 to water in red blood cells under the influence of carbonic anhydrase accounts for about 70% of the CO2 transported from the tissues to the lungs. This CO2 transport route is therefore the most important. In fact, when animals are given carbonic anhydrase inhibitors (such as acetazolamide) to block the action of carbonic anhydrase in red blood cells, CO2 transport from the tissues becomes so poor that tissue Pco2 values can rise to 80 mmHg, higher than normal (normal is 45 mmHg).
3. As (30%) carbamino compounds :-
Transport of Respiratory Gases- CO2 reacts directly with water as well as with amine radicals on the hemoglobin molecule to form the compound carbaminohemoglobin (CO2Hgb). This binding of CO2 to hemoglobin is a loosely bound and reversible reaction, so CO2 is readily released into the alveoli, where PCO2 levels are lower than in the pulmonary capillaries. Similar reactions between small levels of CO2 and plasma proteins occur in the tissue capillaries. Because the amount of these proteins in blood is only one-quarter of the amount of hemoglobin, this reaction is not critical for CO2 transport.
The amount of CO2 that can be transported from peripheral tissues to the lungs by a combination of carbamino with hemoglobin and plasma proteins is about 30% of the total amount transported, i.e. typically about 1.5 ml of CO2 per 100 ml of blood. However, because this reaction is much slower than the reaction of CO2 with water in red blood cells, it is questionable whether this carbamino mechanism transports more than 20% of the total CO2 under normal conditions.
Carbon Dioxide Dissociation Curve :-
In blood, carbon dioxide is carried as a physical solution that binds to hemoglobin, plasma proteins, and water. Carbon dioxide’s partial pressure determines how much of it binds to blood.
The relationship between the partial pressure of carbon dioxide and the amount of carbon dioxide that binds to blood is depicted by the carbon dioxide dissociation curve. According to a normal carbon dioxide dissociation curve, the blood’s carbon dioxide content is 48 milliliters per cent at a partial pressure of 40 mmHg, 52 milliliters per cent at a partial pressure of 48 mmHg, and 70 milliliters per cent at a partial pressure of approximately 100 mmHg.
The Haldane effect is the displacement of carbon dioxide from hemoglobin due to the binding of oxygen to hemoglobin. A blood oxygen level that is too high leads the carbon dioxide dissociation curve to move to the right.
The Haldane effect’s causes When hemoglobin binds to oxygen, it becomes extremely acidic. This causes hemoglobin to lose its carbon dioxide content in two ways:
1. Hemoglobin that is extremely acidic has little propensity to bind carbon dioxide. The blood’s carbon dioxide is eliminated as a result.
2. Acidity causes excess hydrogen ions to be released. The breakdown products of the carbonic acid are the fluid and carbon dioxide. Carbon dioxide is released by the blood into the alveoli.
