Renal Circulation
Introduction :-
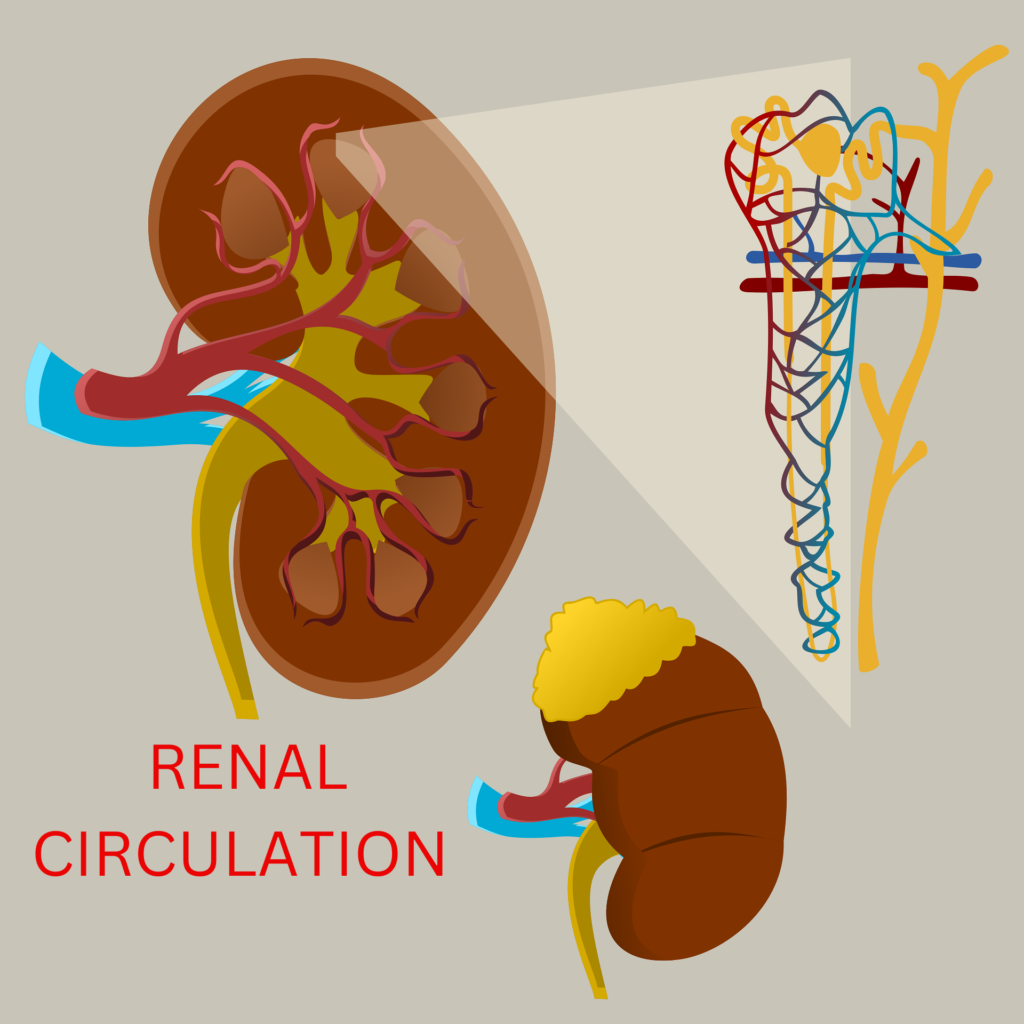
Renal Circulation – The kidney’s blood arteries are highly specialized to support the nephrons’ roles in the production of urine. The kidneys’ maximum blood supply has functional relevance. The kidneys are able to have blood from the renal arteries. Compared to the renal medulla, the renal cortex has substantially more blood flow. Approximately 1,200–1,300 ml of blood, or 25–30% of the total cardiac output, travel through both human kidneys in a minute when the body is at rest and the blood pressure is normal. Although 4 ml of blood circulation per grams of renal tissue has been seen, the pattern of blood distribution to the various kidney sections varies from area to area and is not consistent.
Renal Blood Vessels :-
Renal Circulation – The renal arteries are important both in supplying blood to the kidneys for filtration and in carrying filtered blood away.
Renal arteries :-
Renal Circulation – The renal arteries emerge from the aorta in an almost straight line into the abdominal cavity. Anterior and posterior branches of the renal arteries arise on both sides (70%) at or just before they enter the renal hilum. The renal arteries divide into multiple segmental arteries as they pass through the renal sinus.
1. The division’s major divisions supply the anterior, apical, upper middle, lower middle, lower, and posterior renal vascular segments. We refer to these main branches as segmental arteries. End arteries are typically what these segmental arteries are. One lobar branch for each renal pyramid arises from the division of each segmental artery. The term “interlobar” (straight) arteries refers to the branches that cross between renal pyramids. Arcuate (arciform) arteries are the branches that each interlobar artery produces and which cross the pyramid bases. This arcuate artery system does not communicate with one another. Once more, these arteries produce tiny branches known as small interlobular arteries, which travel radially through the cortex and reach the surface of the kidneys.
2. The interlobular artery divides into many arterioles in instances of superficial cortical glomeruli, the majority of which form an afferent channel to the glomerulus.
3. It is discovered that the afferent arterioles contain a large number of juxtaglomerular cells just prior to entering the glomerulus. Each afferent arteriole splits into roughly 50 capillaries, which remain next to one another to create the kidney’s cortex’s glomerulus. The capillary tuft comes together to create the arteriole that is efferent. This channel divides once more into a second capillary network, known as the peritubular network, which winds around the various sections of the associated renal tubule.
4. The efferent ducts are long and thin, while the afferent vessels are short and wide. This configuration ensures relatively high blood pressure in the glomerulus and improves filtration efficiency.
5. In addition, afferent vessels extend straight and obliquely from the interlobular arteries, facilitating blood flow. The majority of the renal circulation often flows through the superficial cortical glomeruli, which account for approximately 85% of all glomeruli.
Lymphatic supply of Renal Circulation :-
The kidney’s copious lymphatic supply empties into the thoracic venous circulation through the thoracic duct.
Renal lymphatics: The kidneys are equipped with a network of lymphatic vessels that drain excess interstitial fluid, proteins, and waste products from the renal tissue.
Cortical lymphatics: These vessels occur mainly in the renal cortex. They drain the superficial parts of the kidney, especially the glomeruli and the surrounding cortical tissue.
Medullary lymphatics: Compared to the cortical vessels, they are sparsely present and occur in the renal medulla. They drain the deeper parts of the kidney, such as the loop of Henle and the collecting duct.
Lymphatic vessels of the renal capsule: Located in the renal capsule, the outer layer of the kidney. They collect lymph from the surface of the kidney and help drain the renal parenchyma.
Lymphatic drainage pathways: Lymph from the renal cortex and medulla drains into larger lymphatic vessels in the renal capsule.
Autoregulation of Renal Circulation :-
The unique ability of the kidney to always maintain normal blood flow occurs within the physiological limit. The self-regulating system of blood flow is referred to as autoregulation.With the exception of the renal bed, which acts indifferently, the majority of vascular beds adhere to the hemodynamic principle. Pressure in the renal bed increases flow in response to pressure up to 90 mm Hg, and additional pressure increases up to 250 mm Hg will only slightly affect renal plasma flow. The regulation may break down if the pressure is raised above 250 mm Hg.
Mechanism of Autoregulation of Renal Circulation :-
In order to sustain the glomerular filtration rate (GFR), renal autoregulation is crucial. Even in situations where the mean arterial blood pressure fluctuates significantly between 60 and 180 mm Hg, the blood flow to the kidneys stays normal. This aids in preserving a normal GFR. Renal autoregulation involves two different mechanisms:
1. The myogenic reaction
2. Feedback via tubuloglomerular system.
1. The myogenic reaction :-
This idea states that the smooth muscles in the pre-glomerular blood arteries can shorten in response to distension when the perfusion pressure is raised.
Consequently, this myogenic reaction to elevated arterial blood pressure results in an increase in pre-glomerular vascular tone. After paralyzing the smooth muscles with potassium cyanide, papaverine, and a high dosage of procaine, this sensitivity is eliminated.
2. Feedback via tubuloglomerular system. :-
Intrarenal Na feedback theory: This theory posits that the macula densa cells play a crucial role in controlling the release of renin through the glomerular afferent arterioles. Renal blood flow and the rate at which it is filtered out (GFR) will both increase with an increase in blood pressure if it is assumed that the kidneys lack autoregulation.
A higher Na load in the distal convoluted tubule as a result of an enhanced glomerular filtration rate triggers the release of renin from the juxtaglomerular cells. Renin-induced cortical pre-glomerular vasoconstriction will result in a decrease in both the glomerular filtration rate and renal blood flow. Thus, renal autoregulation may be determined by the Na concentration in the distal convoluted tubule at the juxtaglomerular region.
Nervous control of Autoregulation of Renal Circulation :-
The kidneys are supplied with both sympathetic (from the intermediolateral gray portion of the spinal cord, T10 and L2) and parasympathetic (from the vagus nerve) nerves, but have both types of nerve supply. When the renal nerves are at rest, tonic sympathetic discharge is initiated by the cerebral cortex and vasomotor centers. The afferent and efferent arterioles, DCT, PCT, the large ascending limb of the loop of Henle, and the ends of their fibers surrounding the JG cells and renal tubular cells are all sites of sympathetic supply.
